文章管理
Glac Biotech Achieves Another Milestone: Probiotic Strain CP-9 Receives U.S. FDA GRAS Certification
Good news!
Glac Biotech’s core strain, Bifidobacterium animalis subsp. lactis CP-9, has officially received GRAS (Generally Recognized as Safe) certification from the U.S. FDA. Following the success of strain MP108, CP-9 marks Glac Biotech’s second strain to earn this prestigious recognition, making the company one of the few in Taiwan to hold multiple GRAS-certified probiotics.
🌍 Why this certification matters
FDA GRAS certification confirms that CP-9 meets the most rigorous international standards of safety and quality, enabling its use across a wide range of food and beverage applications, including:
Dairy products and plant-based milks
Sports drinks and juices
Cereals and nutrition bars
Infant and toddler foods
This achievement provides Glac’s brand partners with broader, safer ingredient choices — while giving consumers access to more innovative functional food solutions.
🔬 Science-Driven Leadership
In 2023, Glac Biotech’s MP108 became Asia’s first probiotic strain recognized by the U.S. FDA for use in infant foods (GRN001130).
CP-9 has been shortlisted for the prestigious NutraIngredients Awards, with scientific studies validating its benefits in gut health and immune modulation.
With this latest GRAS certification, Glac further strengthens its position as one of Taiwan’s leading probiotic raw material manufacturers trusted worldwide.
🌱 Beyond CP-9: Expanding the Probiotic & Postbiotic Pipeline
Glac Biotech continues to translate scientific data into global health solutions. In addition to CP-9’s GRAS certification, the company is advancing its Totipro® postbiotics across Europe, North America, and Southeast Asia — including regulatory submissions — to meet rising international demand for premium-quality postbiotics.
🚀 Looking Ahead: Science and Partnerships
Since its founding in 2008, Glac Biotech has focused on strain development, patent strategy, clinical validation, and international certifications. The GRAS recognition for CP-9 not only represents years of investment, but also opens new doors for collaboration with leading global brands.
In the years ahead, Glac Biotech will continue to:
Validate and expand the applications of its probiotic and postbiotic portfolio
Broaden its range of GRAS-certified strains and postbiotic solutions
Deepen partnerships with global brands and OEM clients to co-high-quality products that meet international standards for safety and efficacy
Media Coverage
-
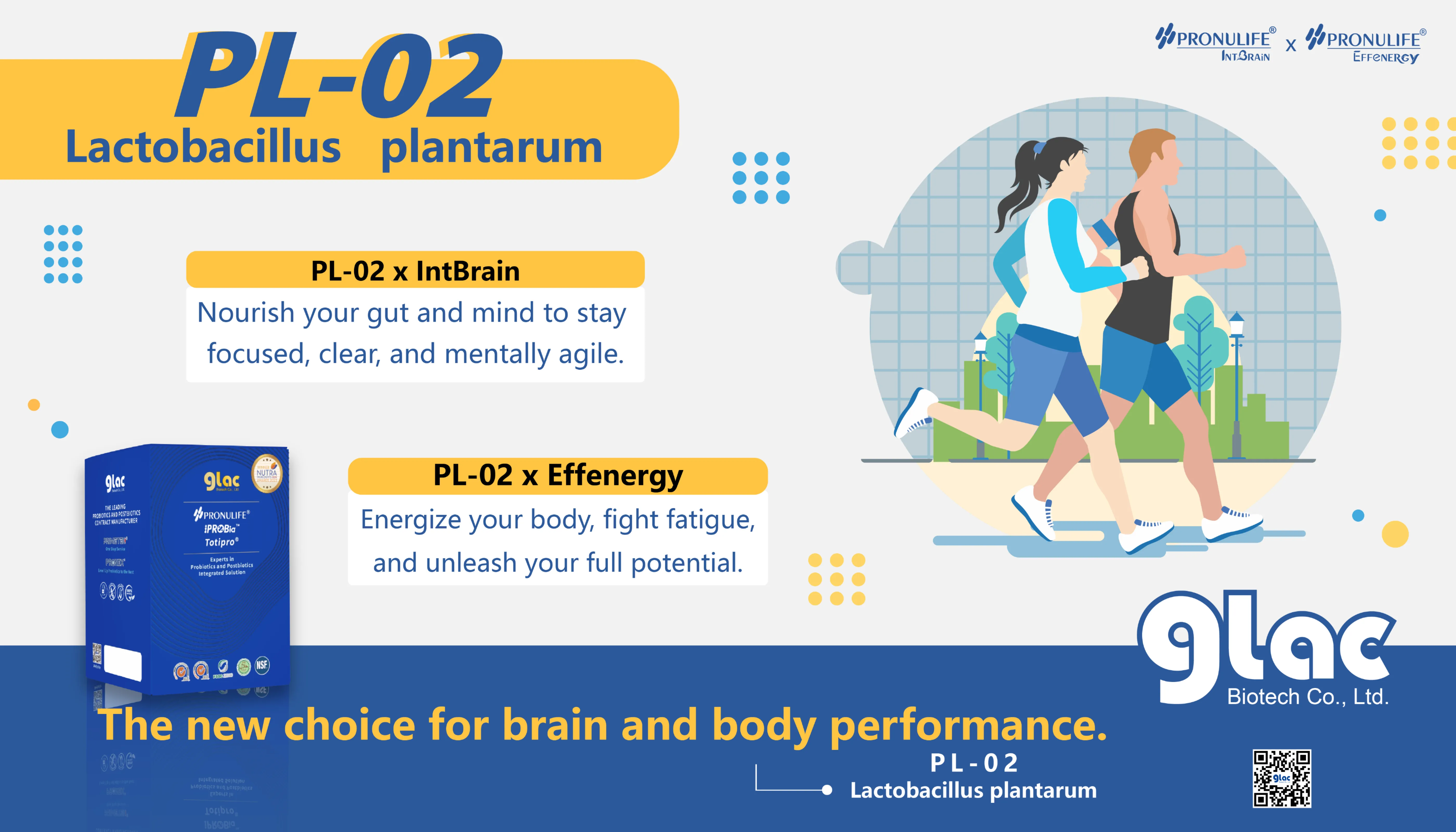
Glac Biotech Wins Double Honors at the iSEE Global Innovation Awards
Lactobacillus plantarum PL-02 Sets a New Benchmark for Functional Probiotics
-

2025.08.07 Taiwan’s First GRAS-Certified Probiotic — Glac Biotech’s MP108 Achieves Historic U.S. FDA Recognition
-

2025.7.9 Glac Biotech Donates Probiotics to Support Vulnerable Communities in Changhua

